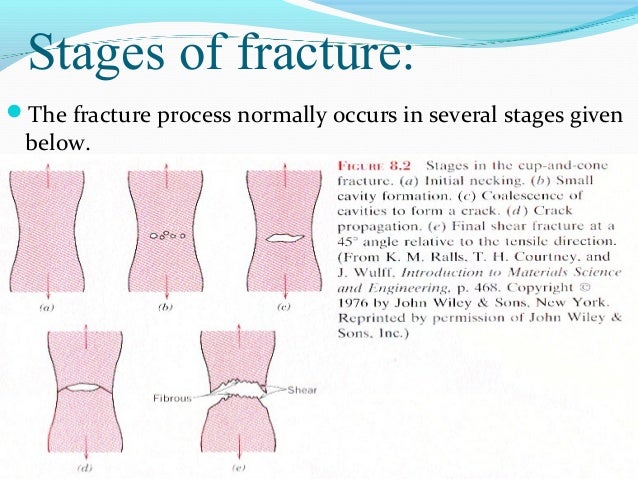
Load Brittle And Ductile Crack Propagation
The approach taken in linear elastic fracture mechanics is to estimate the amount of energy needed to grow a preexisting crack in a brittle material. HELP, HEDE, particles, EBSD, microstructure, EBSD, texture. Phenomenologically hydrogen embrittlement is the result of the absorption of hydrogen by susceptible metals, leading to the delayed or sudden loss of ductility and reduction of load bearing. Stress loading even far below the macroscopic yield point of the embrittled material can result in cracking and sudden or delayed catastrophic brittle failure. Hydrogen embrittlement is. It belongs to the class of physics based corrosion phenomena. It is one the most complex materials phenomena because hydrogen is very. Hydrogen embrittlement of steels and related transition metall alloys is attributed to a variety of possible mechanisms. At room temperature, hydrogen atoms can be absorbed into the metal lattice. The absorbed hydrogen may be present either as an atomic or in recombined molecular form. Regardless of the form, the atoms or molecules. These bubbles can act as pressure concentrators, building up pressure between the metal grains. The pressure can increase to levels where the. The cracking is intergranular. Michael_Berthaume/publication/291832085/figure/fig11/AS:322163946082309@1453821278132/Figure-8-Crack-resistance-curves-R-curves-for-fracture-of-a-brittle-and-b-ductile.png' alt='Load Brittle And Ductile Crack Propagation' title='Load Brittle And Ductile Crack Propagation' />NFPA 59AProposed 2016 Edition Standard for the Production, Storage, and Handling of Liquefied Natural Gas LNG TIA Log No. R Reference Various. To receive news and publication updates for International Journal of Polymer Science, enter your email address in the box below. That is, the crack grows along the metal grain boundaries. Another mechanism that is often discussed in that context is that atomic hydrogen can directly segregate to dislocations and grain boundaries, reducing the cohesion of the metallic lattice at. An essential aspect is also that hydrogen can decorate vacancies, substantially reducing their formation energy, hence, the vacancy density can be drastically enhanced upon. These vacancies can promote the formation of nano voids, void clusters at interfaces, or bigger voids that lead to crack initiation. Another mechanism that is often. This leads to locally higher dislocation densities. A further mechanism that seems to be of relevance is that the decoration of hydrogen atoms to dislocations seems to promote also their mobility. Driver For Compaq Keyboard there. Fatigue crack growth is simulated for an elastic solid with a cyclic cohesive zone model CZM. Material degradation and thus separation follows from the current. Concordia University. Load Brittle And Ductile Crack Propagation In SteelUnderstanding hydrogen assisted embrittlement of advanced structural materials is essential for enabling future hydrogen based energy industries. A crucially important phenomenon in this context. Factors affecting the hydrogen embrittlement are the hydrogen content, residual stress as well as applied stress and microstructure. These factors can all lead to a critical condition for hydrogen embrittlement through specific mechanisms, e. In our group at the Max Planck Institut in Dsseldorf we study the mechanisms of hydrogen embrittlement in a new class of steels, namely FeMnC. TWIP steels. These materials present a new group of advanced high strength and formable austenitic steels with high potential for automotive forming applications. Moreover, energy related infrastructures and pipelines are envisaged as future applications of these steels. The hydrogen promoted embrittlement of steels depends on the kind of steel and also on the hydrogen charging conditions. Phases such as soft ferrite, hard C martensite, maraging martensite. Several important mechanisms were proposed Hydride induced embrittlement, i. Hydrogen enhanced decohesion, HEDE, leading to brittle fracture. Hydrogen enhanced localized plasticity, HELP, leading to ductile fracture. Hydrogen enhanced vacancy formation and stabilization through the defectant mechanism. My current understanding of failure in many pipeline and sheet steels relates to the interaction among these different phenomena, characterized by these steps i H enhances dislocation formation leading to high dislocation densities and dense configurations ii the dislocation interactions can create vacancies e. These nanovoids can coalesce and condensate at interfaces, interface junctions, microbands, twins, or cell walls v The voids can grow further to form larger voids. There are three main required aspects that promote failure due to hydrogen embrittlement A susceptible material, typcially a high strength metallic alloy. Exposure to an environment that contains hydrogen and promotes formation of atomic hydrogen. This can be permanent sour gas environment, hydrogen proplled industry cycles or. The presence of internal tensile stresses due to residual andor applied stresses. Hydrogen can enter and diffuse through a metal alloy surface at ambient or elevated temperatures. This can occur during various manufacturing and assembly operations or when the component is in. Typical industry processes that can lead to hydrogen embrittlement include galvanic zinc coating, phosphating, acid pickling, electroplating and arc welding. During these processes, there is a possibility of absorption of hydrogen by the material. For example, during arc welding. During use, hydrogen can be introduced into metal as a result of corrosion, chemical reactions of metal with acids, or with other chemicalsnotably hydrogen sulfide in sulfide stress cracking. Hydrogen embrittlement of austenitic steels is of high interest because of the potential use of these materials in hydrogen energy. In order to elucidate the associated hydrogen embrittlement mechanisms, the mapping of heterogeneities in strain, damage crackvoid, and hydrogen and their. Specifically mapping the connection between microstructure heterogeneityand the associated hydrogen trapping at similar spatial resolution opens a novel pathway to identify hydrogen embrittlement mechanisms in complex alloys. One of the materials classes that is expected to be applied for energy related structure parts are austenitic steels with high Mn content. In particular, twinning induced plasticity TWIP. Mn austenitic steels are well known for an exceptional balance of ductility and strength with less hydrogen susceptibility compared to ferritic steels with a similar. The hydrogen embrittlement phenomenon has been observed under severe mechanical deformation and hydrogen charging conditions such as delayed fracture testing in a deep drawn cup. Fracture was in such cases caused by various metallurgical factors e. The importance of deformation twins on hydrogen embrittlement of austenitic steels has been recently studied. It was found that they can act as crack initiation sites and enable crack. Microstructure sensitive mapping of hydrogen in TWIP steels and generalla in related metallic alloys has been conducted by using microprinting experiments, which enable visualization of. Ag. Br emulsion. The microprinting technique demonstrated that hydrogen is indeed localized at deformation twins. Also, from our previous experiments we suggested that the hydrogen localization atnear deformation twins requires local plastic straining. These facts indicate that the hydrogen assisted twin boundary cracking of TWIP steels is a complex phenomenon including both, local plastic straining and local. To better understand the influence of deformation twins on the hydrogen embrittlement phenomenon, it is thus essential to map the spatial hydrogen distribution through a high. It should be noted here that the microprinting technique is only sensitive to hydrogen at relatively. It has, therefore, to be applied directly after a sample is charged with hydrogen. It will be shown here that hydrogen. Hence, the findings obtained by microprinting needed to be confirmed also for longer times after charging by a more sensitive technique. In the last. years several promising novel approaches have been reported for the localized resolved and sensitive detection of hydrogen. One is a direct electrochemical detection via a capillary cell, developed by Suter et al. Another approach is the use of Kelvin probe. Studies at quite high resolution have been. Scanning Kelvin Probe Force. Microscopy SKPFM, where diffusion profiles of hydrogen have been mapped successfully at relatively highresolution at cross section of samples after hydrogen charging. However, a direct quantification is not possible by this method, due to the complex dependence of the work function of oxide on different defect states in the oxides. For the same reason this. A new approach by.